Abstract
Introduction
Severe complications of ITP in children are rare; however, the knowledge of these events, along with the use of various medications can impact quality of life (QoL). Since the rate of mortality and severe morbidity is low in ITP, patient/ parent proxy-reported outcomes can be valuable in determining disease outcome. The Kids ITP Tools (KIT) is a health-related quality of life (HRQoL) questionnaire that is specific for pediatric ITP. The KIT was developed in 2007 and validated for use internationally in 2013. In 2019, the American Society of Hematology updated its guidelines to recommend a thrombopoietin receptor agonist (TPO-RA) as second-line treatment in pediatric ITP. The purpose of this study was to review and update the KIT to ensure face validity when evaluating QoL for paediatric ITP patients both prescribed and not prescribed TPO-RAs to align with current clinical practice.
Methods
This is the first stage of a two-stage study using a mixed-methods approach. Two groups of children with a diagnosis of ITP and their families were recruited from one quaternary care Canadian pediatric hospital. The TPO-RA group was defined as having started a TPO-RA after March 1st 2017 which is the date these drugs were licensed in Canada for use in children. All others were classified as non-TPO-RA.
Copies of the child-self-report and parent--/proxy-report versions of the KIT were sent electronically via email to participating families. Children ≥ 7 years old were asked to review the child self report form. Parents/Caregivers reviewed the parent proxy report or the parent impact report. Interviews were conducted via telephone with children and parents. During the interviews, participants were asked to identify the level of importance of each question, provide feedback regarding existing questions and identify topics that were missing from the questionnaire. The responses were coded, entered into an excel spreadsheet and analysed. The most and least important questions were identified. When there was discrepancy, the child's perspective was given priority. Changes suggested for deletion or addition were reviewed with experts, who determined the finalized changes to ensure it aligned with current clinical practice.
Results
Twenty-two participants from 15 families participated. The mean age of the child with ITP was 11.4 (1-19) years. The mean age for TPO-RA participants (n=12) was 12.3 (1-19) and for those not on TPO-RAs (n=7) was 10.0 (4-15). Eight females and 11 males participated. Eleven parents reviewed the parent impact report, 5 the parent proxy report and 10 children reviewed the child self report.
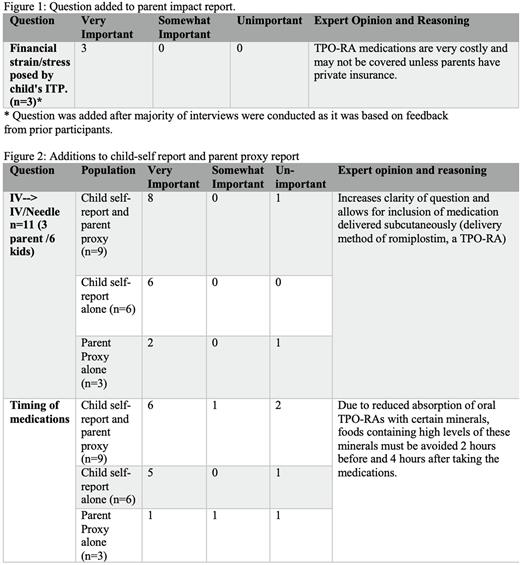
All (100%) participants in the TPO-RA group discussed concerns over the cost of TPO-RA medications; therefore a question was added to the parent impact report, which discusses the financial stress that the child's ITP has on the family (Figure 1). Most (83%) participants on a TPO-RA identified that the timing of medication administration affected their QoL; therefore a question was added to the child self-report and parent proxy report to reflect this (Figure 2). A question discussing impact of treatment through an "IV" was clarified to include the word needle (Figure 2).
One question was removed from the parent impact which discussed feeling unable to relate to other parents who do not have a child with ITP as 9/11 (82%) of parents did not feel that this was an important factor impacting their QoL. A question discussing the burden of staying overnight in the hospital was removed from the child self-report and parent proxy report as 10/15 (67%) classified this as only somewhat important or unimportant. Recent changes in clinical guidelines encourage outpatient treatment of ITP, so admission is much less frequent than in 2007.
Conclusion
The KIT is an effective tool for assessing HRQoL in children with ITP and their families. Due to recent changes in clinical guidelines, the KIT needed to be updated to ensure it is still valid in the era of TPO-RAs. The changes being made used feedback from children with ITP both receiving and not receiving TPO-RAs, their families and clinical experts will ensure that the KIT reflects HRQoL in the current therapeutic environment.
Disclosures
Young:Principia Biopharma: Patents & Royalties. Blanchette:Novo Nordisk: Research Funding; GC Biopharma: Research Funding; Grifols: Research Funding. Klaassen:Principia Biopharma: Patents & Royalties; Agios Pharmacueticals: Consultancy; Amgen: Membership on an entity's Board of Directors or advisory committees; Cangene: Research Funding; Hoffman-La Roche: Consultancy; Novo Nordisk: Consultancy; Octapharma AG: Consultancy; Sanofi: Consultancy; Takeda: Consultancy; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal